Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Characterization of Lesions in Diseased Fishes, Isolation and Identification of Edwardsiella Tarda from Selected Lakes and Ponds of Ethiopia
*Corresponding author: Abdi Feyisa Fufa, Department of Clinical Studies, Addis Ababa University College of Veterinary Medicine and Agriculture, POBox 34, Bishoftu, Ethiopia.
Received: August 25, 2022; Published: August 29, 2022
DOI: 10.34297/AJBSR.2022.17.002301
Abstract
Background: Fish is a nutritious animal rich in protein and consumed than other feeds throughout the world. Although many infectious agents affect fish species, bacterial infections are the known causative agents affecting fish industries.
Methods: A cross-sectional study with purposive sampling was conducted from December 2020 to May 2021 with the objectives of lesion characterization in diseased fishes, isolation, and identification of Edwardsiella tarda (E.tarda) from Batu, Langano, and Babogaya lakes and ponds in Bishoftu.
Results: A total of 60 fishes were examined for this study. Skin, muscle, gill, liver, kidney, spleen, and heart were inspected grossly and collected for microscopic lesion characterization and E.tarda isolation. Out of 60 samples, 28.3% (n=17) and 25% (n=15) were positive for E.tarda by bacteriological and conventional PCR (E.tarda rest at 114bp) respectively. There was significant difference (P<0.05) observed in the frequency of identifying E.tarda between the three study areas and four fish species, but, there was no significant difference between the sex groups (P>0.05). After the histopathological process and staining, tissues were divided into two based on E.tarda confirmation. Gills from E.tarda positive fishes revealed hyperplasia, focal necrosis, and swelling of the lamellar epithelium. Microscopic lesions in the liver include nuclear condensation, cytoplasmic vacuolation, and swelling of hepatocytes. Lesions in the hepatic blood vessels comprise severe hyperplasia of the endothelium, hyperemia, and elastic fibers in the intima of hepatic artery. Kidneys of E.tarda positive fishes had suppurative interstitial nephritis, melano-macrophage deposition in interstitium, and interstitial hemosiderin deposition. There were infiltration of inflammatory cells and focal area necrosis of the epidermis, lymphocyte infiltration in muscle, and accumulation of hemosiderin pigments in spleen. Microscopic lesions in E.tarda negative fishes indicated significant changes like lamellar epithelial degeneration with vacuolation, and total necrosis of structure in gill, periportal hepatitis, and hepato-necrosis with vacuolation of liver, glomerulitis, multifocal tubulointerstitial nephritis, epithelial cells degeneration and cloudy swelling in kidney, dermal edema in skin and myocarditis with fibrosis of heart.
Conclusion: Based on the study several lesions were identified from diseased fishes. The characterized lesions show the occurrences of different diseases in fishes of selected study areas. Thus, further studies on lesion characterization in diseased fishes of different species with large sample sizes and study area should be conducted.
Keywords: Edwardsiella Tarda; Fish Diseases; Haematoxylin and Eosin; Lesion; Lakes; Ponds; Ethiopia
Introduction
Fish are the most diverse groups of vertebrates dwelling in a variety of marine and freshwater habitats. Fisheries continue to be a significant source of food, nutrition, income, and livelihoods for people around the world [1]. Currently fish make up about 19% of the total protein consumption or over the 5% of proteins from both plants and animals origin [2]. Fish consumption in sub-Saharan Africa is the world’s lowest. Ethiopia’s fish production is almost based on inland water bodies like lakes, reservoirs, and rivers [3]. Even if the country has an estimated fish production of 51,481 tons annually, national per capita fish consumption is 0.5 kilogram per year but the recommended quantity is between 12 and 17 kilogram per year [4]. This low per capita and under feed of fish meat is constraint by different factors. The most known constraint is disease (of infectious) agent. Infectious and non-infectious fish diseases are global problems, and they affect freshwater, marine water, and cultured fish [5]. Infectious diseases are caused by pathogenic organisms present in the environment or carried by other fish and are contagious.
Non-infectious diseases are non-contagious, caused by environmental problems, dietary deficiencies, or hereditary anomalies. Infectious diseases are broadly classified as parasitic, bacterial, viral, or fungal diseases [6,7]. Fish are vulnerable to great hazards exerted by parasites and other disease-causing agents especially when they are stressed. Among organs of fishes susceptible to diseases, the skin and gills are leading [8]. Both opportunistic and obligate parasites are the most common cause of infectious diseases in fishes. Parasitic diseases of fish are most frequently caused by protozoa that live in the aquatic environment. A variety of protozoans infest the external part of fish causing irritation, weight loss, and eventually death. Fungal spores are abundant in the aquatic environment, although they rarely cause disease in healthy fish. Fungi colonize damaged exterior tissue of fish after they are infected with an external parasite, bacterial infection, or injured by handling. The injured areas appear to have a cottony growth or appear as brown matted areas when the fish are removed from the water [9,7].
Endo-parasites of fish are common pathogens regularly reported to impose fish production in growing countries including Ethiopia. Trematodes, cestodes, nematodes, and acanthocephalans infect the internal organs of fish with their intermediate stages and sometimes encysting in various host tissues or most adults mainly affect the digestive and circulatory systems of their hosts [10]. But there are very limited studies concerning the economic and public health impact of endo-parasites in Ethiopia [11,12]. The stress conditions like oxygen depletion, poor water quality and overstocking are the factors that stimulate bacterial pathogens in fish. Fish infected with a bacterial disease have hemorrhagic spots or ulcers along the body wall and around the eyes and mouth. Also have an enlarged, fluid-filled abdomen, and protruding eyes during internal infection that requires treatment with medicated feeds containing antibiotics. External bacterial infections result in erosion of skin and ulceration. Columnaris is an example of it which caused by rough handling. The lesions induced by infectious diseases, whether bacteria and/or viral should not be confirmed without a laboratory test [9].
One of the important bacterial diseases in fish is edwardsiellosis associated with Edwardsiella spp [13]. E.tarda bacterium is a known causative agent of a fish disease causing gangrene and septicemia lesions in different organs [14]. The pathogenesis of E. tarda is multifactorial. The intestine and abraded skin are the most likely sites for penetration of the bacteria [15]. E. tarda potential virulence factors include siderophores, cell adhesion factors, and cell invasion activity, and two types of hemolysins [16]. Fish infected with E. tarda exhibit abnormal swimming behavior and floating near the water surface [17], loss of pigmentation, exophthalmia, the opacity of the eyes, swelling of the abdominal surface, petechial hemorrhage in fin and skin, necrotizing small skin lesions and rectal hernia [13,18]. It occurs sporadically in both fish and humans [19,3].
In Ethiopia, major fish-borne bacterial and parasitic zoonoses were studied [20,3]. Studies on internal fish parasites were conducted in Lake Zeway [11,21], in Lake Lugo (Hayke) [22], in Lake Charcher [23]. Isolation of E.tarda from fishes of Lake Zeway and Langano by Kebede and Habtamu [5] and Lake Tana by Nuru [24] has been provided information about the diseases occurrence. However, no research has been conducted on the characteristics of pathological lesions in diseased fish in Ethiopia. In this study we aimed to isolate and identify E. tarda by bacterial cultured, biochemically characterized and, molecularly isolated; additionally we have attempted to characterize the pathology of confirmed E.tarda and negative lesions of E.tarda in diseased fishes from Batu, Langano, Babogaya lakes, and ponds in Bishoftu. To the best of our knowledge, this is the first study carried out to characterize lesions of E.tarda and non-E.tarda infection in fish from Ethiopia.
Materials and Methods
Study Area
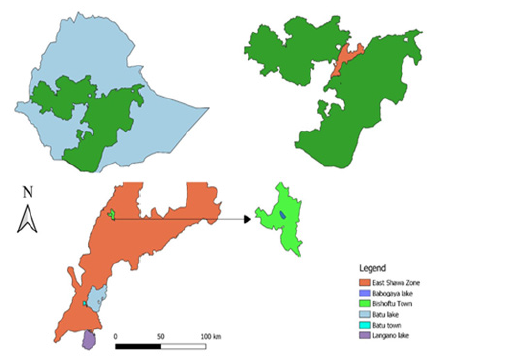
The study was conducted in Batu, Langano and Babogaya lakes and ponds in Bishoftu. Batu Lake is located in Central Ethiopia in Oromia Regional State to the Eastern side of Batu town 163 km southeast of the capital Addis Ababa. It lies in the Northern part of the rift valley between 7°51’N to 8°7’N and 38’43’ E 38°57’ E with an open water area of 422 km2 and shoreline length of 137 km. The lake is fed by two major rivers, Ketar and Meki river and has one outflow in the South, Bulbula river which flows into Abiyata Lake. The lake has several inhabited islands including Tulu Gudo, Tsedecha, Funduro, Debresina, and Galila [25]. Batu Lake is one of the Ethiopian lakes with an indigenous fishing population known for its aquaculture practice with fish ponds managed mainly by local communities. The major fish species in the lake include Nile tilapia, Tilapia zilli, Catfish, Barbus and Carp species [26]. There are many landing points around the lake from where fish is collected either by boat or trucks and brought to the major landing points adjoining Batu town [5].
Lake Langano is located 200 km South of Addis Ababa lying between 7°36’ N and 38°45’ E. It is 18 km long and 16 km wide with an open water area of 230 km2, 7.5 km shoreline and 1600 km2 catchments area. The main fish species in the lake include Barbus species, Clarias species and Oreochronis niloticus with the total annual catch of 1000 tones [27].
Bishoftu town (Figure 1) is located in the East Shewa zone at 47 km South of Addis Ababa. The town is located at 8°45′N longitude and 38°59′E latitude [28]. Lake Babogaya is the volcanic Crater Lake found in the area of Bishoftu town. The Lake is a small, roughly circular and fairly deep lake found at an altitude of 1870m and about 9°N latitude and 39°E longitude (29).
Study Population
The study populations were different fish species from the selected lakes and ponds. Fish species harvested from study lakes and ponds were Nile tilapia, Common carp, Crusian carp, and Catfish. Because Nile tilapia is highly populated species in the study areas, it was the main part of the catch. They were physically examined for their species, sex and all data was recorded accordingly.
Study Design
A cross-sectional study design with purposive sampling of fishes showing any clinical signs was conducted from December 2020 to May 2021. Fishes showing clinical features were selected, physically visualized and palpated to characterize gross lesions
Methods of sampling and sample processing
Gross Examination and Sample Collection:During harvesting from lakes, fishes were examined for any abnormal signs. Fish with clinical signs were safely transported to the nearby laboratory in container with water. On arrival in laboratory, the fishes were put on a clean postmortem examination table and inspected for any visible gross lesions externally, specifically lesions in gill and skin. The eye was covered with a towel, and euthanized humanely by decapitation, and followed by rapid destruction of the brain by pithing.
All necropsy and tissue sampling procedures were carried out under aseptic conditions with aseptic surgical instruments. An incision was made along the midline of the abdomen from the anus to the mouth. Another dissection was made from the anus to the lateral line which continued up to the gills cover to expose the internal organs [5]. The internal parts specifically muscle, liver, spleen, heart, and kidney of fish were visually observed for their consistency, texture and the findings were recorded. Then tissue samples were collected from skin, gill, muscle, liver, spleen, heart, and kidney and contained in the containers with 10% neutral buffered formalin for histopathology and containers with sterile normal saline for E.tarda isolation.
Sample Transportation:The bottles containing the sample were kept in icebox with icepack until transported to Addis Ababa University College of Veterinary Medicine microbiology laboratory where bacterial isolation was conducted. The bottles containing the sample in 10% buffered formalin, were kept in icebox was transported to National Animal Health Diagnosis Center (NAHDIC) pathology laboratory for histopathology work.
Isolation of Edwardsiella Tarda:Tissue samples from skin, gill, muscle, liver, heart, spleen and kidney of freshly killed fish were homogenized in sterile physiological saline. Then the homogenate taken by sterile pipette was dropped on tryptone soya broth and incubated at 37°C for 24 hours. The growth of bacteria was checked for turbidity and cloudy formation in the broth. From broth with turbid and cloudy appearance, a loopfull sample was streaked on xylose lysine deoxycholate agar (XLD) plate and incubated at 37°C for 24 hours. Colonies were characterized for morphology and those with morphological characteristics of E.tarda were further subcultured again on XLD plate and incubated at 37°C for 24 hours to get pure colonies. From the resulting pure culture, primary identification was done based on gram staining, motility, catalase and oxidase tests. Gram-negative, motile, short rods, catalasepositive and oxidase-negative were considered for further tests to confirm whether the colonies belong to E. tarda. Motility test was conducted using Sulfur Indole and Motility Test Media (SIM) by inoculating fresh colony into the medium and incubated at 37°C for 24hr. Outgrowth of bacteria from the stub line was seen [30]. Secondary identification of isolates was conducted by biochemical tests to check the ability of the bacterium to utilize three sugars using Triple sugar iron agar (TSI), use of citrate as a source of carbon for metabolism, an amino acid, or an alcohol source in the medium and production of byproducts detected using appropriate indicators incorporated into the medium [31].
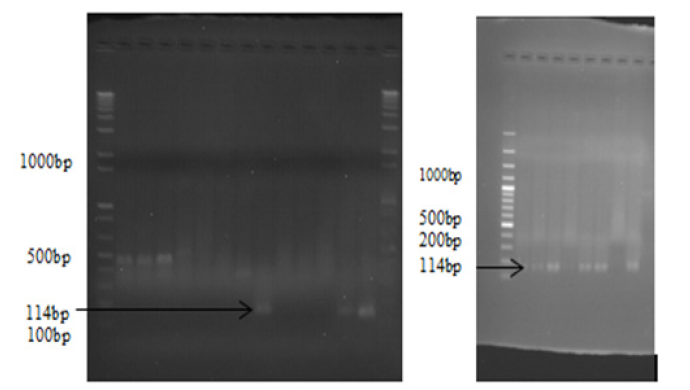
Molecular Identification of Edwardsiella Tarda:After biochemical confirmation, the isolates were preserved by tryptone soya broth with and without paraffin oil for further molecular identification. The PCR was done at National Veterinary Institute (NVI) molecular laboratory. E.tarda species-specific primers were used. Briefly the genomic DNA from 17 isolates was extracted following the manufacturer’s suggested protocols using the DNA isolation kit which includes Taq polymerase, QIAGEN protease, AL buffer, AW1 buffer, AW2 buffer, alcohol and 1.5 ml microcentrifuge tube for extraction. Including the extraction control, 18 QIAamp spin column extractions were prepared. Next to DNA extraction master mix was prepared for molecular analysis using 3μl of Nuclease free water, 2μl of E.tarda forward (ETF) and E.tarda reverse (ETR) from 20μmol of each primer ETF 5’CAGTGATAAAAAGGG GTGGA-3’and ETR 5’CTACACAGCAACGACAACG 3’, 10μl of IQ super mix and 3μl of DNA template given a total of 20μl for one reaction and had a total of 500μl master mix for 25 reactions. After the above two processes the PCR was optimized for scientifically studied thermal conditions of E.tarda for amplification of the genome by varying temperature and exposure time; 95°C for 5 min of initial denaturation followed by 35 cycles of 95°C for 15sec of denaturation, 58°C for 15sec of annealing, and 72°C for 15s of elongation with a final extension step of 72°C for 5 min. Agarose gel was prepared at 1.5% for observation of the amplified DNA. Four [4] μl of gel red was added with loading dye, PCR product and markers or ladder. Aliquots of each amplification reaction (5μl) were electrophoresed through agarose gel by using a ladder having >1000bp each ladder increase by 100bp from the other, and visualized under ultraviolet light for the presence of the appropriately sized bands (ET: 114 bp) [32].
Tissue Processing and Histopathology Techniques:Tissues were trimmed and put into plastic labeled cassettes and processed using an automatic tissue processor. In a processor, the tissues were dehydrated by ascending concentrations of alcohol (70%, 80%, 95%, and 100%), were cleared by xylene and impregnated by molten paraffin. Tissue block was made and from each tissue block, 5μm sections were sectioned using a microtome. The tissue ribbons were made to be floated on a warm water bath at 45°C and the relaxed ribbons were collected on microscopic slides. The tissues ribbons were dewaxed by heat and xylene, hydrated in descending grades of alcohols (100%, 95%, and 70%), and finally stained by Haematoxylin and Eosin (H&E). The stained slides were dehydrated in ascending grades of alcohol (70%, 80%, 95%, and 100%), cleared by xylene and mounted using DPX (Dibutylphthalate Polystyrene Xylene) and examined under a microscope.
Data Analysis
The data generated from field and laboratory investigations were recorded, screened and coded using a Microsoft Excel spreadsheet. The isolation and PCR results were analyzed using statistics (frequencies and percentages). The association between the dependent variable (E.tarda positivity on PCR) and independent variables (sex, fish species, and selected lakes) were analyzed by chi-square using R software. Descriptive statistics of median was used for characterization of lesions.
Results
Gross Lesion Description
Clinically diseased fish were grossly characterized as red operculum [Figure 2A], swelling, congested vent and bleeding of the anus (blue arrow in Figure 2B), hemorrhage on ventral parts of body and swollen abdomen (black arrow in Figure 2 B), tumefaction of eye (white arrow in Figure 2C), white spot on muscle (black arrow Figure 2D) and pale gills (Figure 2E).
Bacteriological Isolation of E.tarda
Samples comprising pulled skin, gill, muscle, kidney, spleen, heart, and liver were collected from 60 clinically diseased fishes. The samples were processed for E.tarda isolation. The isolates appeared as small, whitish colonies with black center on Xylose Lysine Deoxycholate (XLD) agar after 24 hrs. of incubation at 37°C.
E.tarda suspected isolates were undertaken for further biochemical tests. Out of 60 samples collected from fish; samples showed characteristics of E. tarda represented 28.3% (n=17) which were gram-negative short rods, motile, catalase-positive. In biochemical tests, isolates showed various characteristics in different tests (Table 1).
Conventional PCR result of E. tarda
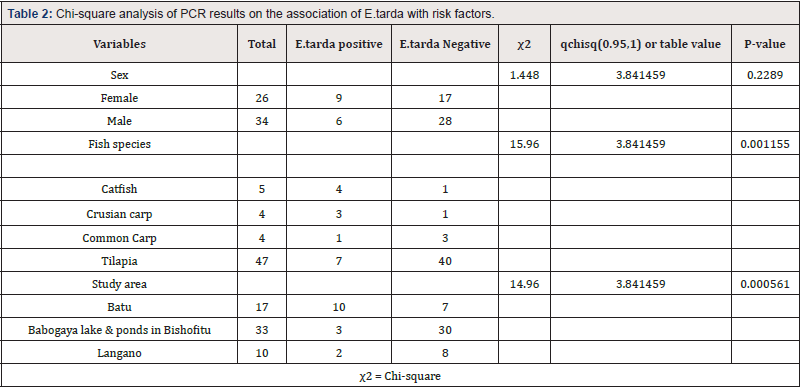
The electrophoresed result was read by ultra-violate light connected by the computer then E.tarda rest at 114bp (Figure 3). Out of 17 biochemically isolated samples, 88.2% (n=15) were deemed as E. tarda positive by PCR which represented 25% of the total 60 samples. About 34.6% (n=9) females and 17.65% (n=6) males were positive for E.tarda. Four species of fishes incorporated in the study, from that about 78.3% (n=47) were tilapia, 6.7% (n=4) crusian carp, 6.7% (n=4) common carp and 8.3% (n=5) catfishes. There was a statistically significant difference (P<0.05) seen in the frequency of identifying E.tarda between the three study areas and four fish species. But there was no statistically significant difference between the sex groups (P>0.05) (Table 2).
Microscopic Lesions In E.Tarda Positive Fishes
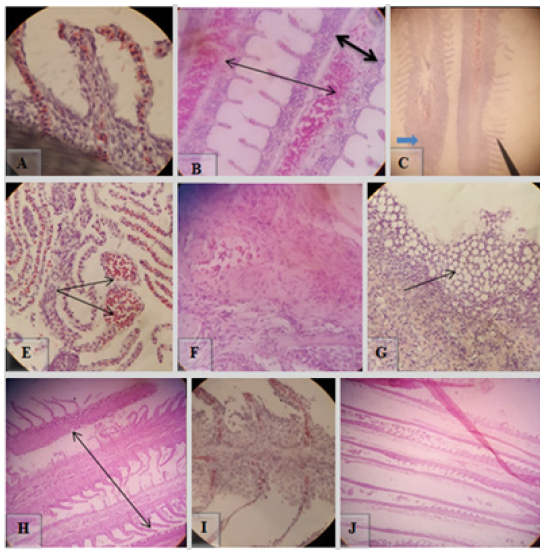
Histopathologically, gills of E.tarda positive fishes were characterized by hyperplasia of gill lamellae (Figure 4A), focal necrosis, and swelling of the lamellar epithelium (black arrow in Figure 4B) and (White arrow in Figure 4B). H&E, 100X (A) & 40X (B).
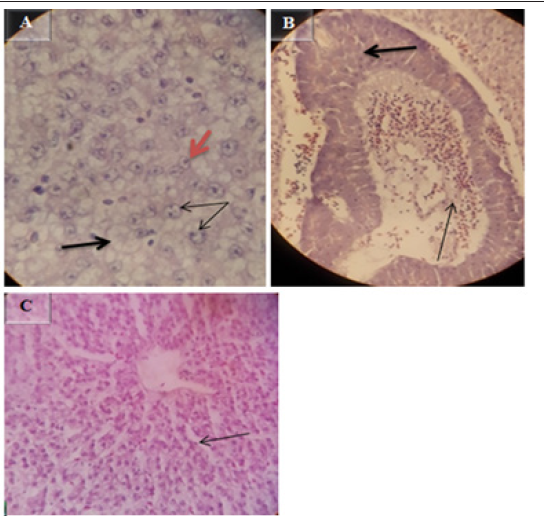
The microscopic lesion in the liver of E.tarda confirmed fish includes necrobiotic changes such as peracinary severe zonal hepatic necrosis with many hepatocytes with nuclear pyknosis or total nuclear loss (orange arrow in Figure 5A), and dilation of hepatic sinusoids (Figure 5C), swollen hepatocytes with peripheral pulled nucleus ( double arrow in Figure 5A), hepatocytic cytoplasm vacuolation (thick black arrow in Figure 5A), Examination of blood vessel revealed severe hyperplasia of the endothelium (thick arrow in Figure 5B), and hyperemia and elastic fibers in the intima of hepatic artery with many layers of fibers in the arterial walls (thin arrow in Figure 5B). H&E at magnification power of 40X.
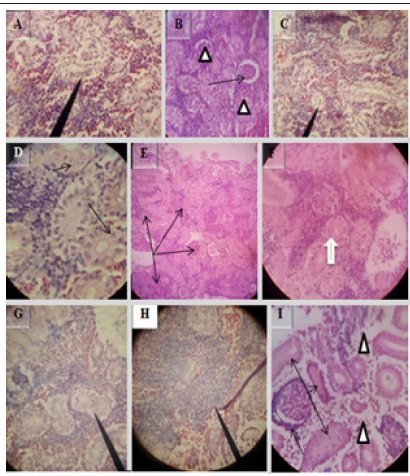
5Histopathological examination of E.tarda positive kidney revealed suppurative interstitial nephritis (Figure 6A), necrosis with fibrosis of the interstitium (Figure 6B), interstitial hemosiderin deposition (Figure 6D), and edema (Figure 6E). Melano-macrophage deposition in the kidney interstitium (Figure 6C), dilation of bowman’s space due to loss of the epithelium (Figure 6F), hypertrophy of renal tubules (thick arrow in Figure 6G), tubular cytoplasmic degeneration with vacuolation (double arrow in Figure 6G) and congestion of renal blood vessels with multi-focal hemorrhages (Figure 6H). H&E, 10X (A),40X (C, D, F & H) and 100X (B, E & G).
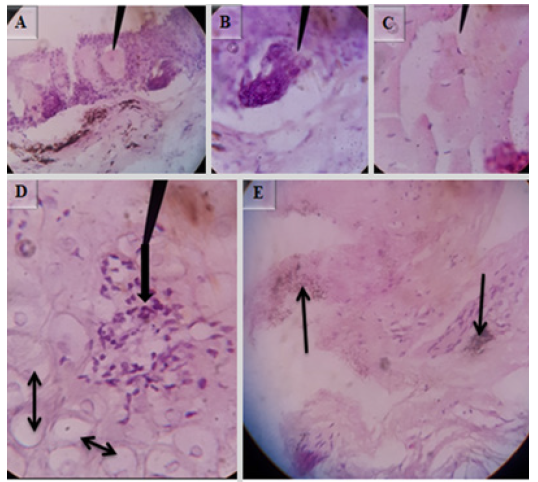
Microscopic lesions in muscle of E.tarda positive fish revealed lymphocyte infiltration (Figure 7A) and lesions in skin include severe dermatitis with infiltration of inflammatory cells into epidermis, focal areas of necrosis in epidermal layer and necrosis of the epidermal layer (Figure 7B). H&E (40X). Microscopic lesions in the spleen include excessive accumulation of hemosiderin pigments around periarterial sheath (thin arrow in Figure 8A&B) and necrotic depletion of the lymphoid tissue in the spleen (thick arrow in Figure 8B). H&E (40X).
Microscopic Lesions in E. tarda Negative Fishes
Microscopic changes observed in gill were lamellae with dilated marginal channel and dilation of central venous with blood congestion (Figure 9A&B), lamellar cell hyperplasia (blue arrow in Figure 9C), telangiectasis (Figure 9E), lamellar epithelial necrosis, and lifting (black arrow in Figure 9C). The most frequent microscopic changes in gill were fibrosis in lamellar epithelium, epithelial degeneration with vacuolation, necrosis with severe inflammatory cell infiltration of lamellar epithelium and total necrosis of the gill structure (Figure 9F,G,H,I,J). H&E, 100X (A, B & F) and 40X (C, E, G, H, I & J).
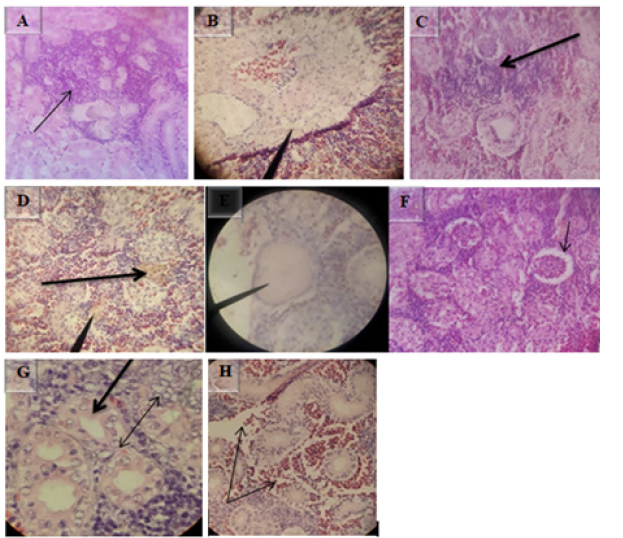
Microscopic examination of liver exhibited centrilobular hepatic necrosis (Figure 10A), congestion of sinusoid (Figure 10B), periportal hepato-necrosis with necrotized hepatocytes around the portal area with infiltration of inflammatory cells (Figure10C), periportal hepatitis, and infiltration of inflammatory cells (thick arrow in Figure 10D), multifocal hepato-necrosis with vacuolation (thin arrow in Figure 10D). Microscopic lesions observed in bile duct were biliary duct hyperplasia (white arrow in Figure 10E), infiltration of inflammatory cells into sub-epithelial lamina (black arrow in Figure 10E), and in some cases loss of bile duct epithelium (Figure 10F). H&E, 40X (A, C, E &F) and 10X (B & D).
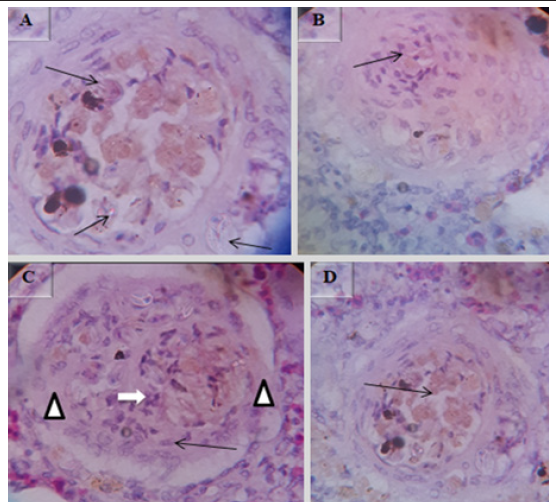
Various microscopic abnormalities were observed in the kidneys. The most frequent changes found in the glomerulus were glomerular expansion resulting in absence of Bowman’s space (Figure 11A), glomerulitis (arrowheads in Figure 11B), destruction of glomerular capillaries (arrow in Figure 11B), inflammatory cell infiltrated around glomerulus (arrow in Figure 11I). In the tubules the most frequent alterations were tubuloepithelial necrosis with epithelial nucleus loss and vacuolation (Figure 11C), hypertrophy of tubular cell nucleus (black arrow in Figure 11D), epithelial cells degeneration (white arrow in Figure 11F), the tubular epithelium lost nucleus; some with pyknotic nucleus others with total loss of epithelium (Figure 11G), degeneration of epithelium and the damaged epithelium form hyaline casts in the tubules (three arrows in Figure 11I). There was severe multifocal tubulointerstitial nephritis with inflammatory cells infiltration (arrows in Figure 11E), the interstitial space infiltrated by inflammatory cells (Figure11 H), total loss of kidney interstitium (arrowheads in Figure11I), H&E, 100X (D), 40X (A, B, C, F, G, H & I) and 10X (E).
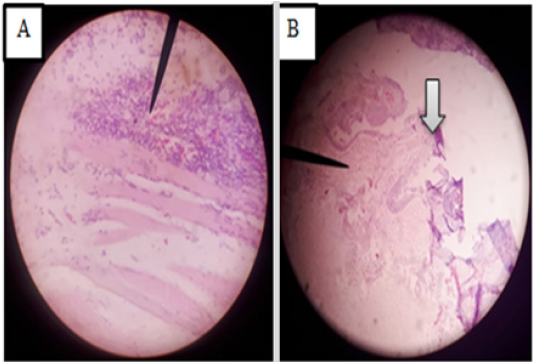
The microscopic alterations seen in the skin were hyperplastic superficial mucus gland with mucus accumulation (Figure 12A), dermal edema (Figure 12C), focal inflammatory nodules in the dermis (single arrow in Figure 13D), dermal cell degeneration, and vacuolation (double arrows in Figure 12D), melanin deposition in the dermal region (Figure 12 E). In some cases cross sectional parasitic cut was observed in the epidermis (Figure 12B). H&E, 100X (B, C, D & E) and 40X (A).
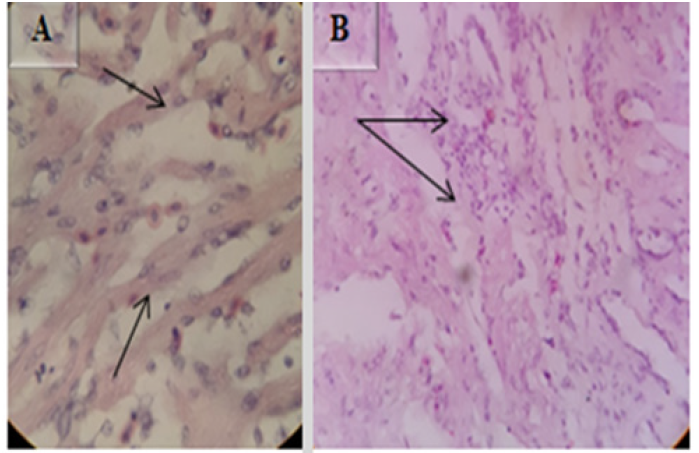
Muscle showed inflammatory myositis with inflammatory cells infiltrated into the endomysium (Figure 13A), capillaries in endomysium were dilated and full of RBC’s, and also there were multifocal hemorrhages (Figure 13 B and C). H&E, 40X (B & C) and 10X (A). Microscopic lesions in the cardiac muscle were severe chronic myocarditis with fibrosis (Figure14A) and heavy infiltration of inflammatory cells (Figure 14 B). H&E 40X.
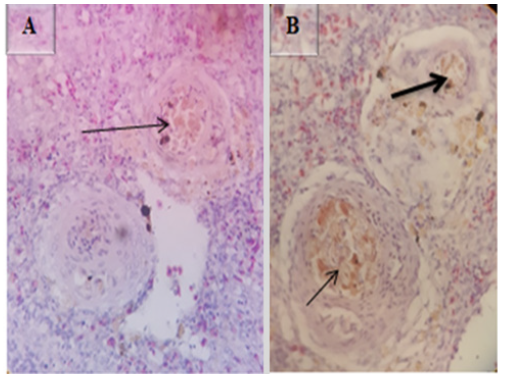
In some cases the spleen showed protozoan parasites with peer shaped sporocysts (Figure 15A), Surrounding these protozoan parasites there were typical multifocal granulomatous lesions (arrow in Figure 15B and white arrow in Figure 15C) with central necrosis (Figure 15D) followed by lymphocytic layers (arrowheads in Figure15 C) into which are intermingled by epitheloid macrophages (black arrow in Figure 15C) H&E (100X).
The fishery sector plays a significant role in food security through the supplementation of protein source food for all population of the world’s, particularly developing countries [2]. One of the problems of the fishery sector in both natural environments and in culture is the disease which has a serious impact on fish [33].
In the present study the microscopic lesions in diseased fishes were characterized, E. tarda was bacteriologically isolated and molecularly categorized. Grossly examined lesions in this study like pale gills, tumefaction of eye and red operculum, swelling and bleeding of the anus, hemorrhage on ventral parts of body, swollen abdomen and congested vent, which were agrees with report undertaken in South Korea by Oh et al. [34] and his collaborators described: “upon visual inspection of the diseased fish, there were no significant external lesions that are commonly observed, but proptosis was evident, which is a typical clinical symptom of bacterial infection”. As compared to the reports of studies conducted in India by Abraham et al. [35] and Hoque et al. [36] most of the gross lesions were indications of bacterial diseases.
According to Ibrahem et al. [37] the gross lesions caused by edwardsiellosis from experimentally infected Nile tilapia and African catfish in Egypt were described as scale detachment and pale discoloration, severe edematous swelling at the site of injection, swollen abdomen with yellowish ascetic fluid, protruded hemorrhagic anus with opaqueness eyes. Internally, both Catfish and Nile tilapia showed severe hemorrhagic enteritis with adhesion between organs, the body cavity was filled with abundant yellowish mucoid fluid; in catfish, the liver showed multiple tiny white foci while the intestine contained thick white opaque mucus. The current study doesn’t observe most gross lesions due to the possible reasons of variation in way and severity of infection, fish species and environmental factors.
In this study, E. tarda was isolated from pulled samples of skin, gill, muscle, kidney, spleen, heart, and liver of 60 diseased fishes indicating that 17 fishes (28.3 %) tested positive by colony characteristics and biochemical tests and 15 (25%) were confirmed positive through PCR test. The study showed a significant association between fish species and E. tarda which is contrary to the previous report by Kebede and Habtamu [5], it may be due to the small sample size used in the recent study which may not be sufficient enough to show such differences. But there was no significant differences in the frequency of identifying E. tarda between sex groups which agree with the earlier studies by Kebede and Habtamu [5], Adanech and Temesgen [31] and also support the agreement with other works were both sex are equal chance of being infected by E.tarda [38,39].
Tissues of E. tarda confirmed fishes were also further processed for microscopic lesion characterization. The lesions in gill were agreed with the previous findings by Darwish et al. [40] and Abraham et al. [35] who studied experimental infection with E.tarda in channel catfish and pathology of E.tarda infection in African catfish, Clarias gariepinus revealed necrosis, hyperplasia, and swelling of lamellar epithelium. These changes could reduce the surface area for effective respiration, which severely stresses fish, or can even lead to death from lack of oxygen.
The findings in liver and kidney were comparable with studies reported by Miwa and Mano [41] stated infection with E.tarda causes hypertrophy of liver cells and enlargement of their nuclei in the Japanese flounder Paralichthys olivaceus, Abraham et al. [35], indicated focal necrosis with lymphocyte infiltration and expansion of space between hepatic sinusoids in liver and neutrophil infiltration, hemosiderin deposition, fibrinoid necrosis of nephretic tubules, and edema in kidney of E. tarda infected catfish. Comparable with lesions reported by Darwish et al. [40] suppurative interstitial nephritis and increased melano-macrophage deposition in interstitium were observed in the kidney and hepatitis in liver. According to Agius and Roberts [42] melano-macrophage centers, also known as macrophage aggregates, are distinctive groupings of pigment-containing cells. It usually contains a variety of pigments, including melanin, and these increase in range and volume in older fish or the presence of cachectic disease. Melano-macrophage centers act as focal depositories for resistant intracellular bacteria
Similar lesions were reported by Hoque et al. [36] who studied on examination of lesions in Pangasianodon hypophthalmus with E. tarda revealed dilation of Bowman’s space, hypertrophy, necrosis of glomerulus and tubular epithelium leading to complete necrosis and fibrosis of renal tubules leading to loss of their normal shape. Vacuolation due to degeneration of cytoplasm and different necrobiotic changes such as cellular and cloudy swelling degeneration of renal tubules in the kidney and blood congestion and necrobiotic changes such as nuclear pyknosis in liver. Accord with observations of Ibrahem et al. [37] microscopic lesion in kidney showed congestion of renal blood vessels with multi-focal hemorrhages.
E.tarda confirmed fish showed lymphocytic infiltration in muscle and severe dermatitis with infiltration of inflammatory cells into epidermis, focal areas of necrosis in epidermal layer of skin were similar with investigations reported by Ibrahem et al. [37] and Abraham et al. [35]. Similar to Darwish et al. [40] stated earlier the spleen revealed an accumulation of haemosiderin pigments these occur due to massive hemolysis of the red blood cells engulfed by macrophages with the presence of hyperemia and necrotic changes.
Microscopic lesions in the gill, skin, muscle, kidney, liver, spleen and heart of E.tarda negative fishes were also studied in the present investigation. Microscopic changes seen in gill agreed with the previous study reported by Camargo and Martinez [43] who studied histopathology of a Neotropical fish caged in an urban stream included dilation of the marginal channel, hyperplasia of the epithelial cells with lifting of the lamellar epithelium and blood congestion. This study is also in agreement with reports of microscopic changes in fish exposed to aluminum and lead acetate by Hadi and Alwan [44] and Mustafa et al. [45] showed lamellar epithelial necrosis and telangiectasis in the gill. The alterations not reported in other studies but frequently seen in these findings were fibrosis in lamellar epithelium, epithelial degeneration with vacuolation, and total necrosis of the gill structure.
The organ most linked with the detoxification and biotransformation process is the liver, and due to its function, position and blood supply [46], it is also one of the organs most affected by contaminants in the water [47]. Microscopic lesions in liver of E. tarda negative fish were agreed with the report by Mustafa et al. [45] who studied on fish exposed to Lead Acetate exhibited various alterations include centrilobular hepatic necrosis and multi-focal hepato-necrosis with vacuolation. Pacheco and Santos [48] described increased vacuolization of the hepatocytes as a signal of degenerative process that suggests metabolic damage, possibly related to exposure to contaminated water.
As Kotob et al. [49] stated that the necrosis in the fish liver can be caused by several factors such as bio-agents (viruses, fungi, bacteria, and parasites), and blood transport disorder that affects the blood supply to particular tissues. All of these disturbances can trigger specific damage to the cell. Congestion of sinusoid and hepatitis with infiltration of inflammatory cells was seen in the study which supported by Naeemi et al. [50] and Maftuch et al. [51]. Periportal hepato-necrosis with necrotized hepatocytes around the portal area and infiltration with inflammatory cells, periportal hepatitis and lesions observed in bile duct were biliary duct hyperplasia, infiltration of inflammatory cells into sub-epithelial lamina and loss of bile duct epithelium were uniquely reported in the study.
The recent microscopic alterations demonstrated in the kidney were supported by the reports of Camargo and Martinez [43] and Hadi and Alwan [44] indicated changes including glomerular expansion causing absence of Bowman’s space, tubuloepithelial necrosis and cloudy swelling, hypertrophy of tubular cell nucleus and hyaline droplet degeneration of Prochilodus lineatus fish caged into organic and inorganic pollutants and freshwater fish Tilapia zillii exposed to aluminum. The tubular epithelium lost nucleus some with pyknotic nucleus others with total loss of epithelium, the interstitial space infiltrated by inflammatory cells, degeneration of epithelium. The finding is comparable with observations of Naeemi et al. [50], Mustafa et al. [45] and Fernandes et al. [52].
The study reported by Maftuch et al. [51], Koi carp due to the infection of Myxobolus sp. parasite revealed inflammatory myositis with infiltration of inflammatory cells in muscle and necrosis and hyaline degeneration in kidney was related with the current finding.
Microscopic changes seen in skin, heart, and spleen that uniquely reported in this study were hyperplastic superficial mucus gland with mucus accumulation, dermal edema, focal inflammatory nodules in the dermis, dermal cell degeneration and vacuolation, melanin deposition in the dermal region, cross sectional parasitic cut in the epidermis, severe chronic myocarditis with fibrosis and the spleen showed protozoan parasites with peer shaped sporocysts, surrounding these protozoan parasites, there were typical multifocal granulomatous lesions with central necrosis followed by lymphocytic layers into which are intermingled by epitheloid macrophage. No earlier study in fish found to compare the findings.
To conclude in present investigation four species of fishes were collected from study lakes and ponds according to the clinical signs shown. E.tarda was bacteriologically isolated, molecularly categorized and microscopic lesions were characterized from E. tarda positive and negative fishes. In the study, non-significant gross lesions of external and internal organs were demonstrated and the most frequent microscopic changes were fibrosis in lamellar epithelium, epithelial degeneration with vacuolation, necrosis with severe inflammatory cell infiltration of lamellar epithelium, and total necrosis in gill, periportal hepato-necrosis in liver, tubuloepithelial necrosis with epithelial nucleus loss and vacuolation and glumerulitis in kidney. Also there were microscopic lesions uniquely reported in the study. This study is the first of its kind to report different microscopic lesions from E.tarda negative fishes in the country. Therefore, further study regarding the different arrays of lesions by combined infection has been isolated works with the trial of isolating all micro-organisms and chemicals that may trigger lesion formation should be implemented. Since E.tarda is important fish and human diseases attention should be given for it and alternative methods to antibiotic treatment are required for prevention and control of the disease in fish farms and aquatic industries.
Data Availability Statement
The datasets generated for this study will be available by the authors, without undue reservation.
Ethics Statement
An ethical certificate with a Reference number of: VM/ ERC/32/06/13/2021 was received from Animal Research Ethics and Review Committee of Addis Ababa University College of Veterinary Medicine and Agriculture. Therefore, the authors guarantee that strict guidelines for handling fish were followed.
Author Contributions
“RW, AF, and JS designed the study. RW performed the data collection and prepared the manuscript. RW, JS, TD analysed the data. JS, AF and YH revised the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version”.
Funding
This work was supported by the Addis Ababa University Vice President for Research and Technology Transfer Office of the Director for Research (Thematic research fund) entitled “Improving meat and carcass quality”.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
Any claims expressed in this article are solely those of authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgment
The authors acknowledges Addis Ababa University College of Veterinary Medicine and Agriculture microbiology, histopathology laboratory staff in laboratory work support and Batu Fishery and Aquatic Life Research Center and staff members for their collaboration in sample collection and guidance. We also thanks National Animal Health Diagnostic and Investigation Center (NAHDIC) histopathology department staff members and National Veterinary Institute (NVI) for their generous cooperation to carry out the molecular laboratory works.
References
- Kerie Y, Nuru A, Abayneh T (2019) Edwardsiella Species Infection in Fish Population and Its Status in Ethiopia. Fisheries and Aquaculture Journal 10(2): 1-6.
- Dugenci SK, Candan A (2003) Isolation of Aeromonas strains from the intestinal flora of Atlantic Salmon (Salmo Salar L 1758). Turkish Journal of Veterinary and Animal Sciences 27(5): 1071-1075.
- Sorsa M, Mamo G, Abera L (2019) Major fish-borne bacterial and parasitic zoonoses in Ethiopia. A review. International Journal of Fauna and Biological Studies 6(4): 50-58.
- Martinus Van der Knaap (2020) As Ethiopians Discover Fish, Innovative Methods Can Improve Consumption. 21: 1063.
- Kebede B, Habtamu T (2016) Isolation and Identification of Edwardsiella tarda from Lake Batu and Langano, Southern Oromia, Ethiopia. Fish Aqua J 7(4): 1-6.
- Nicky BB (2004) Bacteria from fish and other aquatic animals: a practical identification manual. CABI Publishing. 244.
- Aly SM (2013) A review of fish diseases in the Egyptian aquaculture sector: Working report.
- Buller NB (2014) Bacteria and fungi from fish and other aquatic animals: a practical identification manual. Cabi 1-424.
- Verma RK, Saini VP, Dahiya T, Yadav PK (2010) Fish Diseases & Health Management.
- Hodda M (2007) Phylum nematoda. Zootaxa 1668(1): 265-293.
- Lemma A (2013) Study on temporal variation of internal fish parasites in Lake Ziway, Ethiopia. African journal of fisheries science 1(1): 001-004.
- Gebremedhn HG, Tsegay AK (2017) Review on distribution of endo-parasites of fish in Ethiopia. Parasite epidemiology and control 2(4): 42-47.
- Park SB, Aoki T, Jung TS (2012) Pathogenesis of and strategies for preventing Edwardsiella tarda infection in fish. Veterinary research 43(1): 67.
- Nakamura Y, Takano T, Yasuike M, Sakai T, Matsuyama T, et al. (2013) Comparative genomics reveals that a fish pathogenic bacterium Edwardsiella tarda has acquired the locus of enterocyte effacement (LEE) through horizontal gene transfer. BMC genomics 14(1): 642.
- Ali MH, Chowdhury FS, Ashrafuzzaman M, Al Nayem M (2014) Identification pathogenecity, antibiotic and herbal sensitivity of Edwardsiella tarda causing fish disease in Bangladesh. Current Research in Microbiology and Biotechnology 2(1): 292-297.
- Mohanty BR, Sahoo PK (2007) Edwardsiellosis in fish: a brief review. Journal of biosciences 32(7): 1331-1344.
- Woo PT, Bruno DW (2011) Fish diseases and disorders. viral, bacterial and fungal infections. CABI.
- Yoo J, Han S, Hong J, Kang BK, Phuong MT, et al. (2019) Effect of water temperature on occurrence of Edwardsiella tarda in olive flounder Paralichthys olivaceus farms in Jeju island. South Korea.
- Evans JJ, Klesius PH, Plumb JA, Shoemaker CA (2011) Edwardsiella septicaemias. Fish diseases and disorders. viral, bacterial and fungal infections 3(2): 512-569.
- Sharma M, Shrivastav AB, Sahni YP, Pandey G (2012) Overviews of the treatment and control of common fish diseases.
- Nigussu F, Tsegaye T, Dessie S (2017) Prevalence of Contra Caecum Parasite Infestation of Nile Tilapia and African Catfish in Lake Ziway, Ethiopia. J Fish Aqua Dev 2017(4): 120.
- Amare A, Alemayehu A, Aylate A (2014) Prevalence of internal parasitic helminthes infected Oreochromis niloticus (Nile Tilapia), Clarias gariepinus (African Catfish) and Cyprinus carpio (Common Carp) in Lake Lugo (Hayke), Northeast Ethiopia. Journal of Aquaculture Research and Development 5(3): 233.
- Gebawo T (2014) Seasonal variation of parasites of fishes in Lake Charcher west Hararghe, Oromia region, Ethiopia. Nigerian Journal of Fisheries 11: 1-2.
- Nuru A (2007) Study on bacterial pathogens of fish in Southern Gulf of Lake Tana with special references to Aeromonas hydrophila and Edwardsiella tarda. Addis Ababa University 10-30.
- Anonymous (1999) Regional Government of Oromia. Oromia economic study project office. Agricultural sector study draft final report. Addis Ababa 123-135.
- Yimer E (2000) Preliminary survey of parasite and bacterial pathogen at lake Batu. SINET: Ethiop J Sci 23(1): 25-33.
- (1993) LFDP Lake Fisheries Development: fisheries base line survey. Lake Ziway. Working Paper No. 7 Addis Ababa. Ministry of Agriculture 134-165.
- Birhanu M, Leta S, Mamo G, Tesfaye S (2017) Prevalence of bovine subclinical mastitisand isolation of its major causes in Bishoftu Town, Ethiopia. BMC Res Notes 10(1): 767.
- Lemma AH (2012) The breeding saeason and condition factor of oreochromis niloticus (pisces: cichlidae) in lake babogaya, Ethiopia. Pastoral Livestock Systems: Opportunities and Challenges as a Livelihood Strategy 119.
- Zhou Y, Geng Y, Wang KY, Huang XL, Chen DF, et al. (2016) Edwardsiella tarda infection in cultured Ya‐fish, S chizothorax prenanti, in China. Aquaculture Research 47(7): 2349-2354.
- Adanech BH, Temesgen KG (2018) Isolation and identification of Escherichia coli and Edwardsiella tarda from fish harvested for human consumption from Zeway Lake, Ethiopia. African Journal of Microbiology Research 12(20): 476-480.
- Griffin MJ, Ware C, Quiniou SM, Steadman JM, Gaunt PS, et al. (2014) Edwardsiella piscicida identified in the southeastern USA by gyrB sequence, species-specific and repetitive sequence-mediated PCR. Diseases of aquatic organisms 108(1): 23-35.
- Wood AP, Dixon AB (2002) Sustainable wetland management in Illubabor Zone: research report summaries. The University of Huddersfield and Wetland Action, Huddersfield, Kirklees.
- Oh WT, Jun JW, Kim HJ, Giri SS, Yun S, et al. (2020) Characterization and Pathological Analysis of a Virulent Edwardsiella anguillarum Strain Isolated From Nile Tilapia (Oreochromis niloticus) in Korea. Frontiers in veterinary science 7: 14.
- Abraham TJ, Mallick PK, Adikesavalu H, Banerjee S (2015) Pathology of Edwardsiella tarda infection in African catfish, Clarias gariepinus (Burchell 1822), fingerlings. Fisheries & Aquatic Life 23(3): 141-148.
- Hoque F, Pawar N, Pitale P, Dutta R, Sawant B, et al. (2020) Pathogenesis and expression profile of selected immune genes to experimental Edwardsiella tarda infection in iridescent shark Pangasianodon hypophthalmus. Aquaculture Reports 17: 100371.
- Ibrahem MD, Shaheed B, Yazeed HAE, Korani H (2011) Assessment of the susceptibility of polyculture reared African catfish and Nile tilapia to Edwardsiella tarda. J Am Sci 7(3): 779-786.
- Yu L, Yuan L, Feng H, Li S (2004) Determination of the Bacterial Pathogenic Edwardsiella tarda in Fish Species by Capillary Electrophoresis with Blue Light Emitting Diode Induced Fluorescence. Electrophoresis 25(18-19): 3139-3144.
- Savan R, Kono T, Itami T, Sakai M (2005) Loop-mediated isothermal Amplification: an emerging technology for detection of fish and shellfish pathogens. J Fish Dis 28(10): 573-581.
- Darwish A, Plumb JA, Newton JC (2000) Histopathology and pathogenesis of experimental infection with Edwardsiella tarda in channel catfish. Journal of Aquatic Animal Health 12(4): 255-266.
- Miwa S, Mano N (2000) Infection with Edwardsiella tarda causes hypertrophy of liver cells in the Japanese flounder Paralichthys olivaceus. Diseases of aquatic organisms 42(3): 227-231.
- Agius C, Roberts RJ (2003) Melano‐macrophage centres and their role in fish pathology. Journal of fish diseases 26(9): 499-509.
- Camargo MM, Martinez CB (2007) Histopathology of gills, kidney and liver of a Neotropical fish caged in an urban stream. Neotropical Ichthyology 5(3): 327-336.
- Hadi AA, Alwan SF (2012) Histopathological changes in gills, liver and kidney of fresh water fish, Tilapia zillii, exposed to aluminum. International Journal of Pharmacy & Life Sciences 3(11).
- Mustafa SA, Al Faragi J, Salman N, Al Rudainy A (2017) Histopathological alterations in gills, liver and kidney of common carp, Cyprinus carpio exposed to lead Acetate. Adv Anim Vet Sci 5(9): 371-376.
- Van der Oost R, Beber J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environmental Toxicology and Pharmacology 13(2): 57-149.
- Rodrigues EL, Fanta E (1998) Liver histopathology of the fish Brachydanio rerio after acute exposure to sublethal levels of the organophosphate Dimetoato 500. Revista Brasileira de Zoologia 15: 441-450.
- Pacheco M, Santos MA (2002) Biotransformation, genotoxic and histopathological effects of environmental contaminants in European eel (Anguilla Anguilla L.). Ecotoxicol Environ Saf 53(3) : 331-347.
- Kotob MH, Menanteau Ledouble S, Kumar G, Abdelzaher M, El Matbouli M (2017) The impact of co-infections on fish: a review. Veterinary research 47(1): 98.
- Naeemi A, Jamili S, Shabanipour N, Mashinchian A, Shariati Feizabadi S (2013) Histopathological changes of gill, liver and kidney in Caspian kutum exposed to Linear Alkylbenzene Sulfonate.
- Maftuch M, Sanoesi E, Farichin I, Saputra BA, Ramdhani L, et al. (2018) Histopathology of gill, muscle, intestine, kidney, and liver on Myxobolus sp.-infected Koi carp (Cyprinus carpio). Journal of parasitic diseases 42(1): 137-143.
- Fernandes CE, Marcondes SF, Galindo GM, Franco Belussi L (2019) Kidney anatomy, histology and histometric traits associated to renosomatic index in Gymnotus inaequilabiatus (Gymnotiformes: Gymnotidae). Neotropical Ichthyology 17(4).







 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.